Abstract - Short-range DNA looping has been proposed to affect promoter activity in many bacterial species and operator configurations, but only few examples have been experimentally investigated in molecular detail. Here we present evidence for a metal-responsive #DNA condensation mechanism controlled by the Helicobacter pylori ferric uptake regulator (Fur), an orthologue of the widespread Fur family of prokaryotic metal-dependent regulators. H. pylori #Fur represses the transcription of the essential arsRS acid acclimation operon through iron-responsive oligomerization and DNA compaction, encasing the arsR transcriptional start site in a repressive macromolecular complex. A second metal-dependent regulator NikR functions as nickel-dependent anti-repressor at this promoter, antagonizing the binding of Fur to the operator elements responsible for the DNA condensation. The results allow unifying H. #pylori metal ion homeostasis and acid acclimation in a mechanistically coherent model, and demonstrate, for the first time, the existence of a selective metal-responsive DNA compaction mechanism controlling bacterial transcriptional regulation.
Introduction
DNA condensation and looping are fundamental mechanisms for genome biology and gene regulation in prokaryotes and eukaryotes. In bacteria, long-range DNA compaction and gene expression impact on the topological organization of the nucleoid1, while local DNA bending by transcription factors and nucleoid-associated proteins (NAPs) has provided early paradigmatic evidence for the importance of DNA looping in the regulation of promoter elements2, 3.
Accordingly, short-range DNA looping has been proposed to account for particular operator configurations and promoter responses in countless reports and many bacterial species. Nevertheless, only few examples have been experimentally investigated in molecular detail, principally, but not exclusively, involving phage repressors4, and the sugar, nitrogen or nucleotide catabolism regulation in Escherichia coli5, 6, 7, 8. The capability to constrain and to locally distort the DNA is also a key feature of NAPs and global regulators involved in transcriptional control9. Remarkably, the involvement of transcription factor-mediated DNA looping in the transduction of metal-dependent transcriptional responses has not been reported to date, although several metal ions and metal-sensing regulators (such as Fe2+-Fur and Ni2+-NikR) have fundamental roles in bacterial viability, virulence and host-pathogen interactions10. A unique exception pertains to the recent prediction of a putative Fur-dependent DNA looping at the fepB-entCEBAH promoter, inferred from theoretical binding site annotations of E. coli promoters6. Fur proteins belong to an ubiquitously conserved superfamily of prokaryotic regulators involved in the homeostasis of different metal ions and oxidative stress responses11. Because of their ability to oligomerize in a metal-dependent fashion12, 13 and to bind promoters at multiple sites14, 15, 16, they represent ideal candidates for the investigation of metal-dependent DNAe. In addition, topological modifications of the DNA induced by Fur binding, such as bending and wrapping, have been reported in vitro using various footprinting and microscopy techniques, including atomic force microscopy (AFM)17, 18.
Herein, we use the thoroughly characterized H. pylori Fur regulator as a model to explore the metal-dependent short-scale DNA compaction mechanisms involved in transcriptional regulation. To this aim, we investigate the Fur-regulated arsR promoter by a combination of DNase I footprinting, AFM and promoter functional analysis. The rationale behind the choice of this promoter derives from the ChIP-chip and functional evidence that Fur regulates and targets the arsRS operon in vivo19, 20. This operon encodes a two-component system that controls transcription of many H. pylori pH-dependent genes through the activity of the auto-regulated ArsR response regulator21. ArsR is crucial for the positive regulation of the nickel-dependent urease metallo-enzyme, which is important for acid acclimation22. This transcriptional regulator also appears to positively regulate other essential pH-independent functions of H. pylori because, unlike the arsS histidine kinase gene, arsR deletion mutants have not been attainable to date. Interestingly, the acid tolerance of H. pylori was shown to be impaired in both fur and nikR (nickel-dependent regulator) deletion mutants23, 24, fostering the hypothesis of a shallow regulatory circuit linking metal-ion homeostasis with acid acclimation25.
In this work, we characterize the role of these metal-dependent regulators in the transcriptional control of the essential arsRS operon. We demonstrate the direct wiring of Fur and NikR with the ArsR regulon, and highlight the contribution of DNA compaction to this process. In particular, we show that the arsR promoter sports a complex operator architecture with multiple Fur and NikR operators, bound with different affinities according to the metallation state of the regulators. Evidence is presented that this promoter architecture allows for iron- and Fur-dependent repression (FeOFF) through the compaction of the promoter in a nucleo-protein complex. In the presence of nickel ions, binding of NikR antagonizes Fur binding and DNA compaction thus relieving Fur repression (NikRON). The fundamental implications of this metal-responsive promoter compaction mechanism are discussed, together with its pivotal role in the H. pylori transcriptional regulatory circuit.
ArsR transcription is controlled by Fur and NikR metallo-regulators
To investigate how metal homeostasis regulation could transcriptionally feed into the ArsR regulon, we performed primer extension analyses of the native ParsR promoter using total RNA extracted from exponential phase H. pylori cultures and we compared the responses elicited by treatment with different metal ions (Fe2+ and Ni2+) or iron chelator (Dipy), under different genetic backgrounds (Fig. 1).
In a wild-type background, ParsR transcription was repressed by either iron or nickel, while iron chelation caused de-repression of the promoter, pointing to a prominent role of Fur in arsR regulation. Accordingly, ParsR was constitutively de-repressed in a fur knockout strain, suggesting a prototypical holo-Fur repression in which the iron ion acts as co-repressor (FeOFF). While the slight repression observed after nickel treatment could indicate a repressive role of NikR on ParsR, we observed a generalized decrease of arsR transcript levels in the nikR strain, in which the responses to metal-ions and chelators were maintained. At a first glance, this could be interpreted as a NikR-dependent transcriptional activation of ParsR; however, while the responses in wild-type and fur-deletion mutants proved highly reproducible, the nikR-dependent deregulation of ParsR was more variable. In a fur-nikR double mutant background, the ars promoter remained constitutively de-repressed, excluding the requirement of NikR for full promoter activation, and suggesting that the observed nickel-induced repression is directly or indirectly mediated by Fur, which would be responsible for the transduction of the Ni2+ signal. The documented ability of Ni2+ to substitute Fe2+ as Fur cofactor in footprinting experiments14 supports this interpretation.
These results demonstrate that the essential ArsR acid response regulator is under the transcriptional control of the metal ion circuit regulated by Fur and NikR, with the former acting as metal-responsive master repressor (FeOFF) and the latter as positive modulator of arsR transcription.
Complex architecture of the arsR promoter
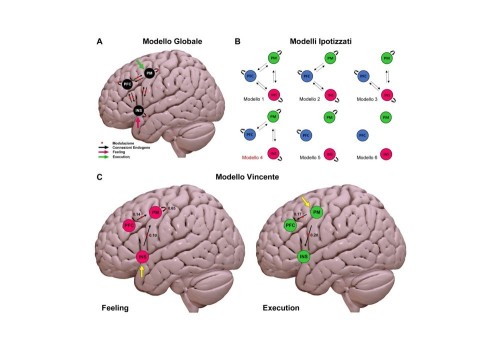
To elucidate the molecular mechanisms responsible for the regulation of arsR, the protein-DNA interactions of recombinant Fur and NikR at the ParsR promoter were investigated by DNase I footprinting (Fig. 2).
Figure 2: DNA footprinting of apo/holo-Fur and apo/holo-NikR at ParsR.
More: http://www.nature.com/ncomms/2016/160825/ncomms125...
Ultimi Articoli
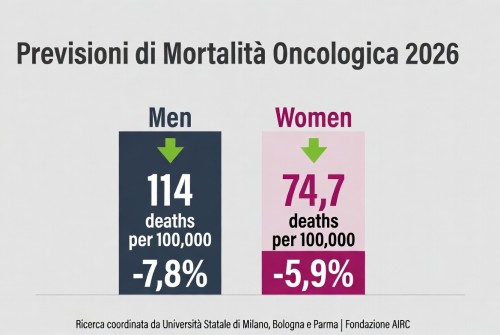
La mortalità per cancro cala in Europa – tassi in diminuzione nel 2026, ma persistono disparità

Carofiglio porta — Elogio dell'ignoranza e dell'errore — al Teatro Manzoni

Teatro per tutta la famiglia: “Inside and Out of Me 2” tra ironia e interazione

Dogliani celebra quindici anni di Festival della TV con “Dialoghi Coraggiosi”

Sesto San Giovanni — 180 milioni dalla Regione per l’ospedale che rafforza la Città della Salute

Triennale Milano — Una settimana di libri, musica, danza e arti sonore dal 20 al 25 gennaio

A febbraio la corsa alle iscrizioni nidi – Milano apre il portale per 2026/2027

Hackathon 2025 — a Palazzo Lombardia gli studenti sfidano il cyberbullismo

Firmato il nuovo Protocollo per il Punto Unico di Accesso tra Municipio Roma III e ASL Roma 1